Innovating Today With A Focus On An Improved Tomorrow
Developing a novel treatment for multiple symptoms of Prader-Willi syndrome (PWS)
Our Innovation
EPM301 (cannabidiol acid methyl ester) is our lead development candidate and was granted Orphan Drug Designation and Rare Pediatric Disease Designation (including a Priority Review Voucher) by the US FDA as well as Orphan Drug Designation by the EU for the treatment of hyperphagia in patients with Prader-Willi syndrome. We are also investigating EPM301's potential to improve other major symptoms of PWS including the sleep/wake cycle and behavioral.
Specializing in
Patient-first Research
epm Therapeutics is a biotechnology company developing cannabidiol acid derivatives for human use. Our singular dedication at this time is researching the potential of our EPM301 to improve multiple and common symptoms of patients with PWS (Prader-Willi syndrome) and how we can potentially improve their quality of life as well as that of their loved ones and their dedicated caregivers.
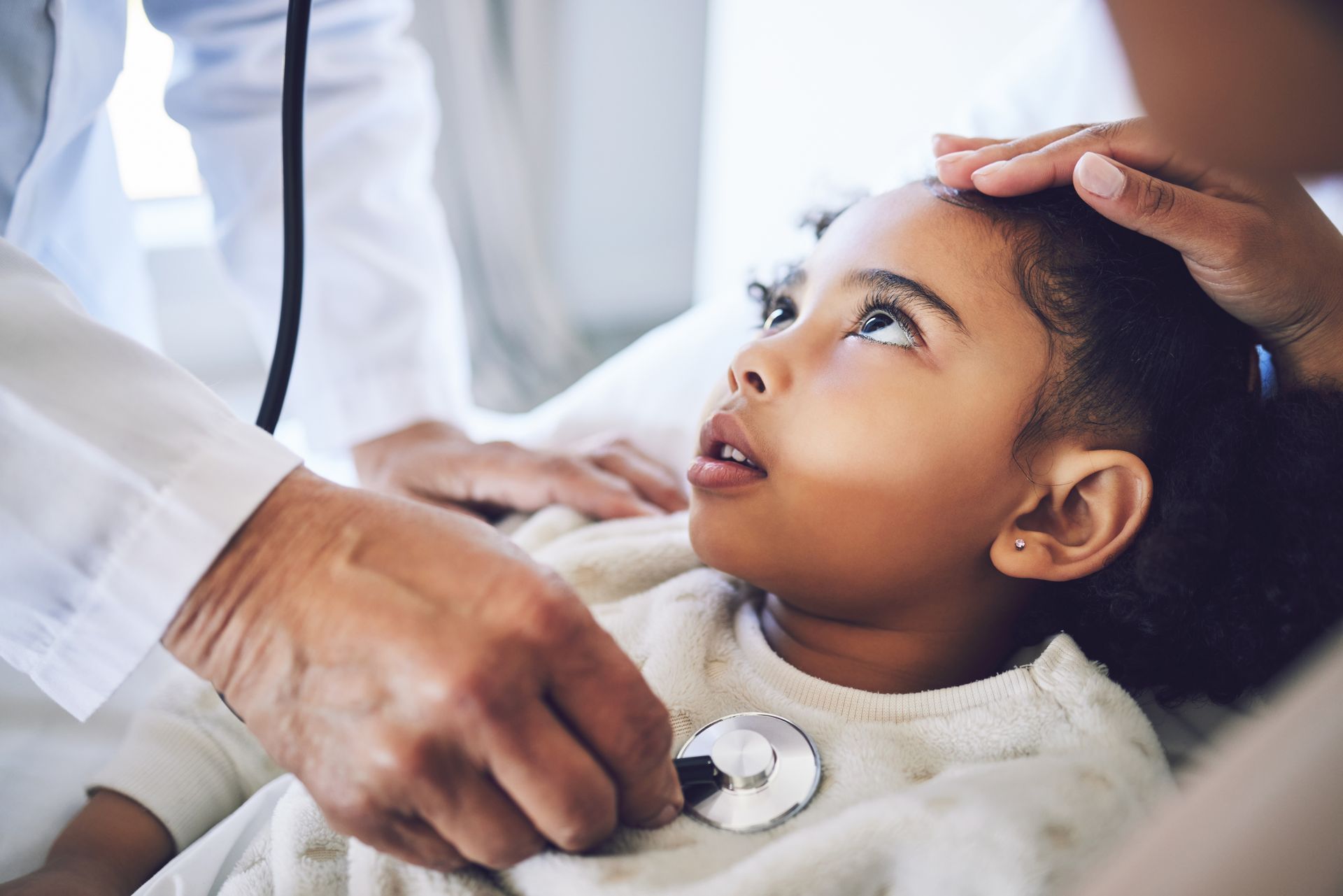
Prader-Willi Syndrome
Prader-Willi syndrome is a rare genetic disorder affecting chromosome 15 that disrupts normal hypothalamic function.

PWS Caregiver Resources
PWS affects the lives of thousands of Americans. There are many resources to help families and caregivers.

Future Developments
We're proud to be part of a growing community of researchers, families, and advocates working toward better solutions.
years of experience
Our Team
epm's management team has over 80 years of global experience in R & D, Financing, Operations and Commercialization of prescription medicines. The team is solely focused on achieving an IND filing for EPM301, proceeding in to the clinic and advancing towards an approval.
Make a Change With Us
We are looking for investors who are committed to building a better tomorrow.
